
The POP02 study continues to enroll participants at an impressive rate. Nearly 15 participants enrolled in POP02 are children with Down Syndrome (DS). Enrolling children with DS in clinical trials helps achieve the goals put forth by INCLUDE.
The purpose of the INCLUDE project is to enhance clinical trial support for medication usage in children with DS and to promote training for clinical trials in those with DS for PTN and DS clinicians.
Specifically, the PTN DS will (1) Characterize the pharmacokinetics (PK), pharmacodynamics (PD), and pharmacogenomics (PGx) of understudied off-patent drugs administered to children and young adults with DS. (2) Develop a stand-alone protocol specifically for those with DS after convening thought leaders and experts. (3) Develop a training program for clinical researchers, both within the PTN and with external DS experts, to provide insight and guidance in trial design, recruitment, and engagement (including assent/consent) issues specifically in this population.
The National Institute of Child Health and Human Development (NICHD) supports this work through the Best Pharmaceuticals for Children Act (BPCA). Dr. Daniel Benjamin, Principal Investigator and Chair of PTN, will partner with Dr. Mara Becker of the Duke Department of Pediatrics as the INCLUDE Principal Investigators. For more information on the NIH’s efforts to support children with Down syndrome and their families, visit DSConnect.


 The Pediatric Trial Network’s (PTN) Digoxin study
The Pediatric Trial Network’s (PTN) Digoxin study
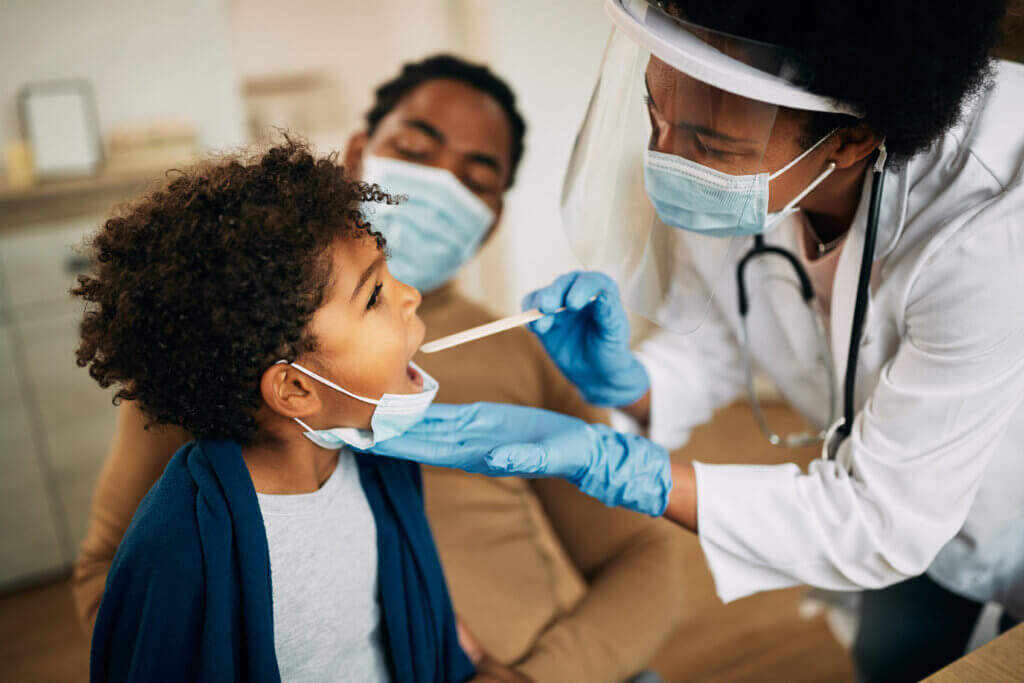

 The Pediatric Trials Network (PTN) has enrolled the first child participant with Down syndrome into the Pharmacokinetics, Pharmacodynamics, and Safety Profile of Understudied Drugs Administered to Children per Standard of Care (POP02) study.
The Pediatric Trials Network (PTN) has enrolled the first child participant with Down syndrome into the Pharmacokinetics, Pharmacodynamics, and Safety Profile of Understudied Drugs Administered to Children per Standard of Care (POP02) study. The Pediatric Trials Network (PTN) Anesthesia and Analgesics in Children (ANA) study has recently closed enrollment for its hydromorphone drug of interest (DOI) cohort (or group of study participants), a major study milestone. This means the goal for participant enrollment for this DOI cohort has been reached for this pharmacokinetic (PK) study, and researchers can study hydromorphone, an analgesic drug, in children.
The Pediatric Trials Network (PTN) Anesthesia and Analgesics in Children (ANA) study has recently closed enrollment for its hydromorphone drug of interest (DOI) cohort (or group of study participants), a major study milestone. This means the goal for participant enrollment for this DOI cohort has been reached for this pharmacokinetic (PK) study, and researchers can study hydromorphone, an analgesic drug, in children. The INCLUDE directive calls for a trans-NIH research initiative to address critical health and quality-of-life needs for individuals with Down syndrome across the lifespan.
The INCLUDE directive calls for a trans-NIH research initiative to address critical health and quality-of-life needs for individuals with Down syndrome across the lifespan.